Creating more options for your patients
Our collection of genomic tests uncover unique insights that empower you to customise treatment for individual patients, throughout their cancer journey.
A different approach to next-generation sequencing
Tumour/normal match DNA sequencing
Tumour/normal matched design provides substantial improvements in mutation identification and a 27.7% reduction in false positive rates compared to tumour-only analysis.1-3
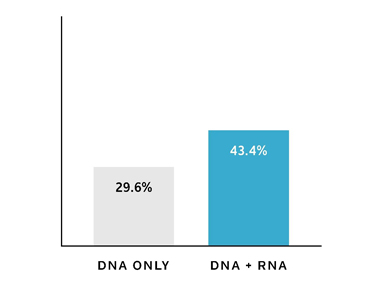
Whole transcriptome RNA sequencing with validated fusion detection
RNA fusion analysis provides clinically-validated fusion detection, matching more patients to high level therapy options when combining RNA, immunotherapy markers and DNA sequencing compared to DNA sequencing alone.1;4
Auto-conversion option to liquid biopsy
In the event of insufficient tumour tissue, you can choose to still receive a blood-based result, through a simple automatic conversion selection, providing genomic results without a delay. In a retrospective study, at least one clinical option was reported for 96% of the cohort on the basis of Tempus NGS and matched clinical data.1
Concurrent testing improves specificity
Concurrent tissue and liquid biopsy testing matches more patients to therapies compared to solid tumour testing alone. Data suggests that just under 10% non-overlapping actionable gene alterations may be detected using concurrent testing.5;6


Longitudinal Testing for greater insight
Longitudinal testing offers insight into the evolution of cancer care throughout the course of disease. Follow-up liquid biopsy testing at disease progression can uncover the following: new gene alterations and resistance mechanisms (clonal evolution); samples tumour DNA shed from metastatic sites; and can be used to longitudinally monitor disease burden and response to treatment.7;8
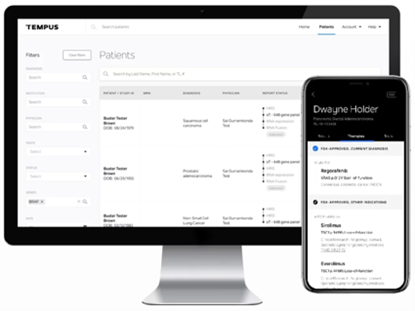
Tempus Hub
Our online Hub allows you secure, fast, reliable access to patient report status and results. View interactive and in-depth results and downloadable PDFs of all Tempus reports.
The Tempus HUB app for phone and tablet users make accessibility even easier. Know the status of testing, notifications of status updates in real-time, interactive report results at your fingertips where ever you are. Request access to the Tempus HUB to have the most advanced smart reporting at your fingertips.
References:
1 Beaubier N, Bontrager M, Huether R, et al. Integrated genomic profiling expands clinical options for patients with cancer. Nature Biotechnology. 2019;37(11):1351-1360. doi:10.1038/s41587-019-0259-z
2 Jones et al. 2015. Sci Trans/ Med. Personalized genomic analyses for cancer mutation discovery and interpretation.
3 Rabizadeh et al. 2018. Oncotarget. Comprehensive genomic transcriptomic tumor-normal gene panel analysis for enhanced precision in patients with lung cancer.
4 Michuda J, 2022, “Clinical whole transcriptome profiling improves the detection of clinically actionable fusions over DNA sequencing alone” paper presented at 2022 ASCO Annual Meeting, Chicago IL June 3-7 2022.
5 Mackay M, 2022, “Dual tissue and plasma testing to improve detection of actionable variants in patients with solid cancers” paper presented at 2022 ASCO Annual Meeting, Chicago IL June 3-7 2022.
6 Finkle JD, Boulos H, Driessen TM, et al. Validation of a liquid biopsy assay with molecular and clinical profiling of circulating tumor DNA. npj Precis. Oncol. 2021;5(1):63.
7 Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non–small cell lung cancer. JAMA Oncology. 2019;5(2):173. doi:10.1001/jamaoncol.2018.4305
8 Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nature Communications. 2016;7(1). doi:10.1038/ncomms11815
Interested in more comprehensive information?
We would be happy to share more detailed information and clinical evidence on Tempus products and services through LINK Medical.
Improving patient care through AI/ML-based algorithms
All AI-based test can be ordered as an add-on with any Tempus xT test, without additional tissue samples.
Pioneering the future of personalised oncology
Comprehensive and complimentary sequencing.
Evidence based approach
Nature Biotechnology Study Reveals that Tempus’ xT Platform Increases Cancer Patients’ Personalized Therapeutic Opportunities
Extensive molecular profiling combined with clinical data identifies targeted therapies and clinical trials for a large proportion of cancer patients, and paired tumor/normal plus transcriptome sequencing outperforms tumor-only DNA panel testing
Validation of a Liquid Biopsy Assay with Molecular and Clinical Profiling of Circulating Tumor DNA
Actionable variants would have been missed if only solid tumor or liquid biopsy tests were performed. Thus, we believe that liquid biopsies provide value to patients when used in combination with standard tissue genotyping.
Prevalence of Germline Findings Among Tumors From Cancer Types Lacking Hereditary Testing Guidelines
This study found that Tumor-normal (T/N) matched next-generation sequencing (NGS) has multiple advantages including enhanced variant classification and incidental germline variant discovery that may be missed with a guidelines-based approach.
Interested in more comprehensive information?
We would be happy to share more detailed information and clinical evidence on Tempus products and services through LINK Medical.
