How can we help you?
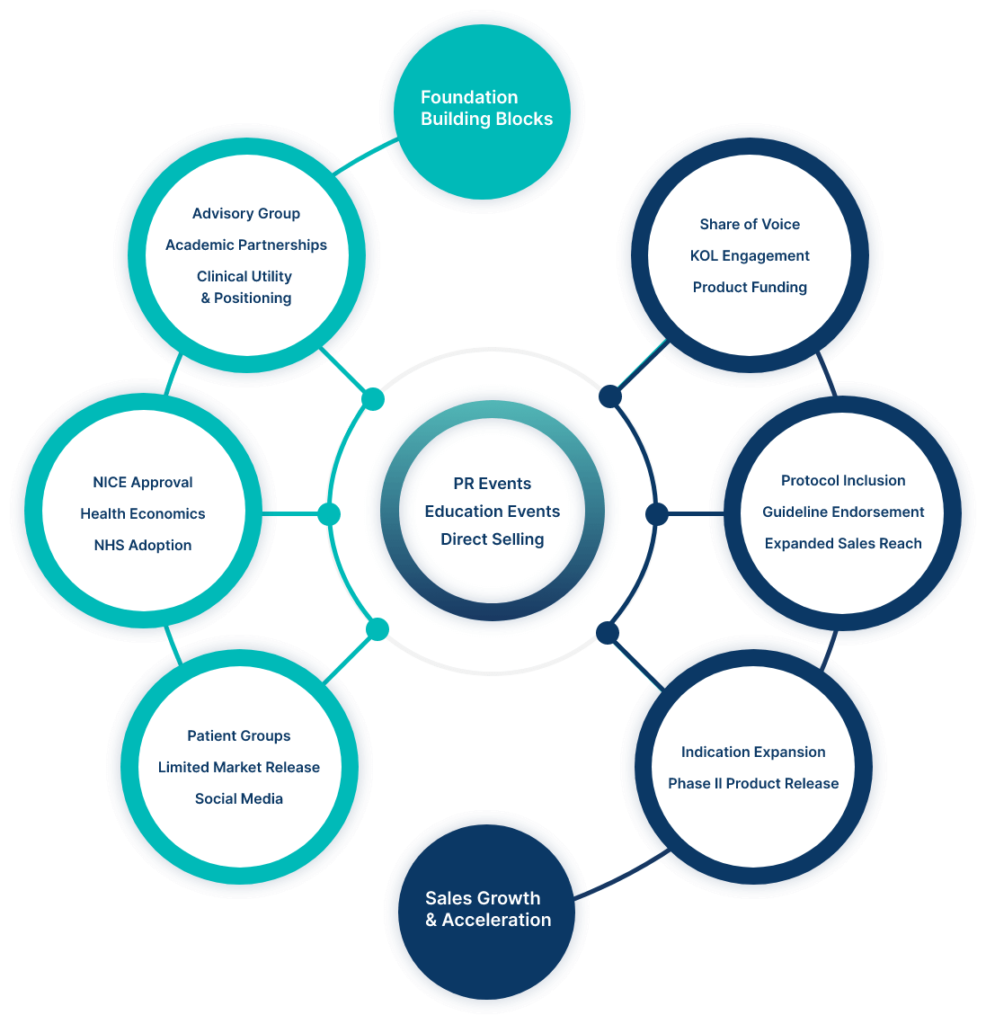
Through our unique modular structure, LINK can support any or all elements required for a successful launch in the U.K. Whether you need assistance with one element or more, we will bespoke our approach accordingly.
LINK work on a very specific payment for delivery model which means that you only pay for what you get and should you decide to stop the project – we will bill for the work delivered up to that point.
Commercialisation of a new technology or product can be highly complex which is why LINK have simplified the process and have gates at each stage to ensure that nothing is missed or left unanswered.